The World Health Organisation sets specific guidelines for the storage of blood , urine and Nasopharyngeal samples with specific temperatures required for each.
Blood collection for serum by venipuncture and handling
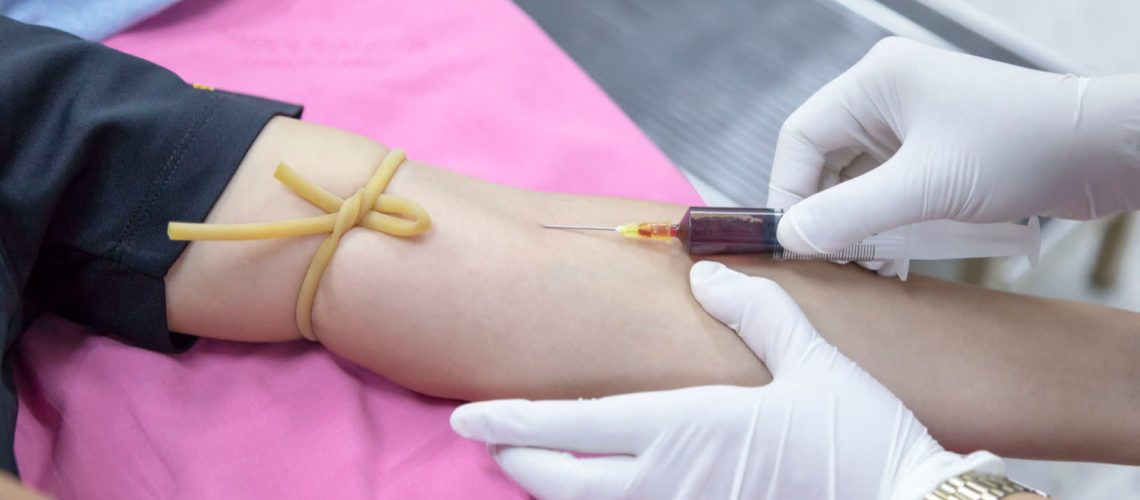
Blood should be collected in a sterile tube (5 ml for older children and adults and 1 ml for infants and younger children) and labelled with the patient’s name and/or identification number and the collection date.
Whole blood can be stored at 4–8°C for up to 24 hours before the serum is separated, but it must not be frozen.
Whole blood should be allowed to clot and then centrifuged at 1000 × gravitational units (g) for 10 minutes to separate the serum. If there is no centrifuge, the blood can be kept in a refrigerator (4–8°C) until there is complete retraction of the clot from the serum (no longer than 24 hours).
The serum should be carefully removed with a fine-bore pipette to avoid extracting red cells, and transferred aseptically to a sterile vial labelled with the patient’s name or identifier, date of collection and specimen type.
A measles/rubella laboratory request form should be fully completed when the specimen is collected and must accompany all specimens sent to the laboratory
Storage and shipment of serum samples
Serum should be stored at 4–8°C until shipment takes place, or for max. 7 days.
When kept for longer periods, serum samples should be frozen at −20°C or lower and transported to the testing laboratory on frozen ice packs. Repeated freezing and thawing of serum samples for IgM testing should be avoided, as it may have detrimental effects on the stability of IgM antibodies.
As a general rule, serum specimens should be shipped to the laboratory as soon as possible. The shipment should not be delayed for the collection of additional specimens.
Serum specimens, in their uniquely labelled, sealed vials, should be placed in sealable plastic bags or pouches containing absorbent materials such as cotton wool to soak up any leakage that may occur.
Styrofoam boxes or an insulating (vacuum) flask should be used to contain the sealed bags or pouches. The specimen form and investigation form for each specimen should be placed in a separate plastic bag and taped securely to the inner surface of the top of the styrofoam box or the outside of the vacuum flask.
If ice packs (which should be frozen) are used, they should be placed at the bottom and along the sides of the styrofoam box. The samples should then be placed in the centre and more ice packs placed on top.
A shipping date should be arranged between the sample collectors and the laboratory. When arrangements have been finalised, the addressee should be informed of the time and manner of transportation. More details on the packaging and transportation of samples are provided in the Manual for the laboratory diagnosis of measles and rubella virus infection.
Storage and shipping of oral fluid
Once a sample has been collected, the device should be sealed according to the manufacturer’s instructions.
If the daily ambient temperature is below 22°C, samples should be shipped to the laboratory within 24 hours.
At higher temperatures samples should be kept in a refrigerator (4–8oC) until they can be shipped to the laboratory on ice.
The samples are usually not considered biohazardous and can be shipped without special requirements or special documentation from the site of collection to the laboratory.
Collection of urine samples
It is preferable to obtain the first urine passed in the morning. Urine (10–50 ml) should be collected in a sterile container and held at 4–8°C before centrifugation.
Urine must not be frozen before the concentration procedure is carried out. A refrigerated centrifuge is recommended, but otherwise start with urine that has been chilled at 4°C.
Urine should be centrifuged at 500 × g (approximately 1500 rpm) at 4°C for 5–10 minutes, preferably within 24 hours after specimen collection. The supernatant should be discarded and the sediment resuspended in 2–3 ml sterile transport medium, tissue culture medium or phosphate-buffered saline.
If centrifugation facilities are not available, whole urine can be shipped directly to the laboratory in well-sealed containers at 4°C immediately after collection. Do not freeze.
Storage and shipping of urine samples
The resuspended pellet may be stored at 4°C and shipped within 48 hours to a measles reference laboratory.
Alternatively, it may be frozen at −70°C or lower in viral transport medium and shipped on dry ice in a well-sealed screw-capped vial to protect against CO2 contamination.
Storage and shipping of nasopharyngeal samples
Nasopharyngeal specimens should be refrigerated and shipped at 4–8°C to arrive at the testing laboratory within 48 hours.
If arrangements cannot be made for rapid shipment, swabs should be shaken in the medium for elution of the cells and then removed.
The medium or nasal aspirate should be centrifuged at 500 × g (approximately 1500 rpm) at 4°C for five minutes and the resulting pellet resuspended in cell culture medium.
The suspended pellet and the supernatant should be stored separately at −70°C or lower and shipped to the testing laboratory on dry ice in well-sealed screw-capped vials to protect against CO2 contamination.
Storage and shipment of whole blood
Whole blood samples may be shipped in well-sealed tubes at 4°C.
EDTA-supplemented whole blood should be processed for virus isolation within 48 hours after collection and must not be frozen at any time prior to processing.